Charge of Nh3 Ligand
NH3 is a Lewis base. The lone pair of electrons can be split when the NH3 atom accepts a hydrogen proton becoming NH4 ammonium.
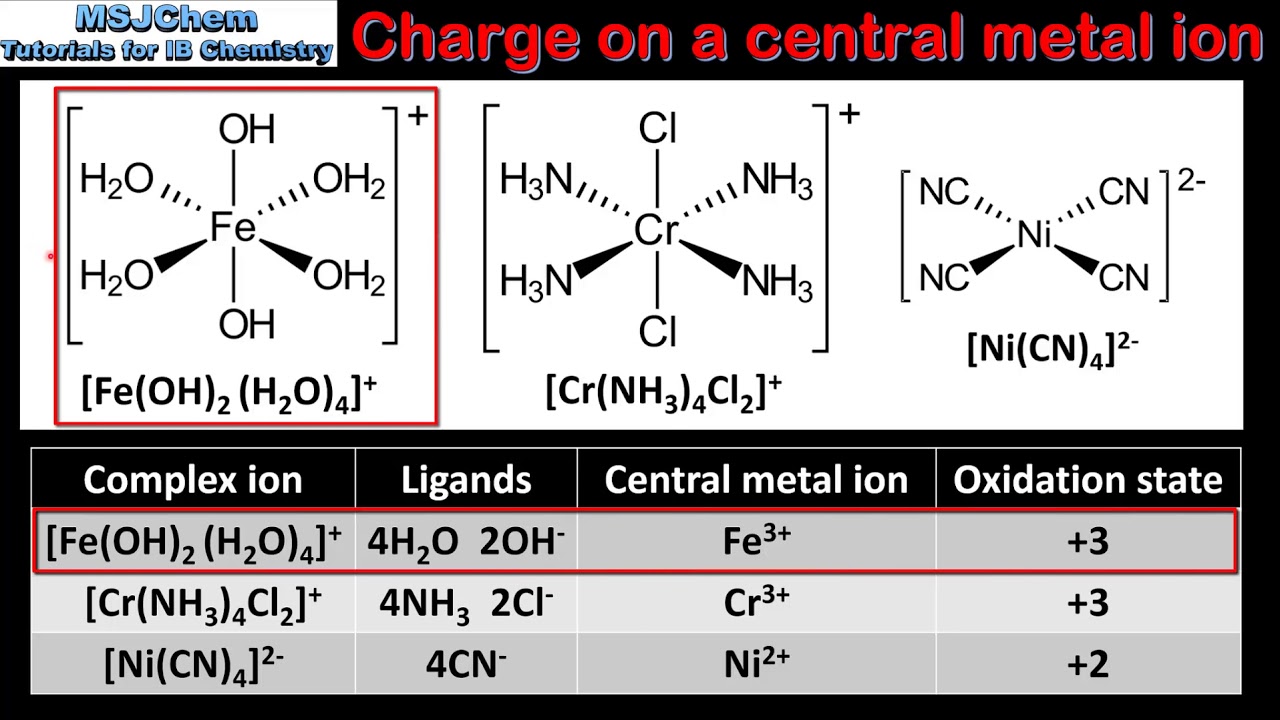
13 1 Deduce The Charge And Oxidation State Of A Central Metal Ion Hl Youtube
Does N in NH3 ligand gains formal positive charge as N in ammonium ion.

. So its N-factor is 1. Karan Nagrale Asks. Traditional solid-state effects such as ligand pi-pi overlap or.
The formal chemical charge of Ammonia NH3 is zero it doesnt actually have a chemical charge. Because of the lone pair of electrons in nitrogen ammonia serves as a ligand. NH3 accepts H ion.
Formally Ammonia NH3 does not possess a charge. NH3 is a ligand with medium field strength. NH3 is a neutral compound therefore the charge of NH3 is zero.
This is because its sigma donating capability is not very strong. Chemists often represent ligands as spheres for simplicity even though the sphere sometimes has three-dimensional structure of its own. Yet until this happens the presence of the lone pair.
The NH3 oxidation numberis the sum of individual oxidation numbers of the atoms nitrogen oxidation number -3 and. Likewise what type of ligand is nh3. While its easy to just say that its important to place that answer in context and.
Ammonium ion is formed when N in ceNH3 forms a coordinate covalent. Why is NH3 neutral. As it has one lone.
Coordinate bonds with the lone electron pair can be easily formed. Why NH3 is a ligand. The ammine ligand NH3 is neutral so only the counter ions will determine the charge.
For example when chemists draw the structure for. 22-Bipyridine is a bidentate. It contains a lone pair of electrons which can go away when NH3 accepts a proton to from NH4.
In other words only one pair of electrons can be donated to the metal ion. The charge on the metal in the complex compound is determined by the charges on the ligands and any counter ions. What is the charge on.
Type Charge Ligand Formula Name in Complexes monodentate neutral ammonia NH3 ammine water H2O aqua carbon monoxide CO carbonyl pyridine pyr pyridine minus one azide N3 azido. So if for Co NH35Cl is the ligand and it is bonded to Cl then we know Cl. In the solid state some of these complexes exhibit split low-energy ligand-to-metal charge-transfer LMCT bands.
Neutral Ligands those having no negative charge on them but have an excess of. Likewise which is strongest ligand.

Coordination Compounds Does N In Nh3 Ligand Gains Formal Positive Charge As N In Ammonium Ion Chemistry Stack Exchange
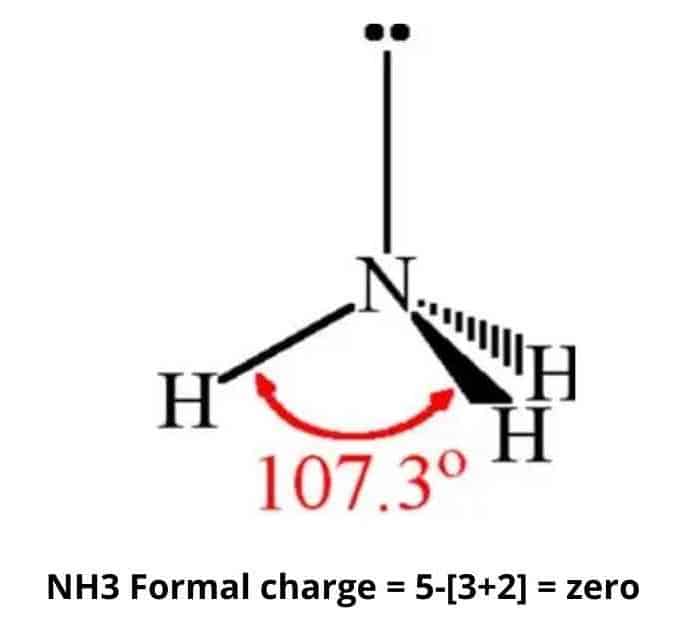
Charge Of Ammonia Nh3 Simple Steps What S Insight

How To Find The Oxidation Number For N In Nh3 Ammonia Youtube

Coordination Chemistry Notes Chemistry Notes Transition Metal Chemistry

Coordination Compounds Does N In Nh3 Ligand Gains Formal Positive Charge As N In Ammonium Ion Chemistry Stack Exchange

Coordination Compounds Does N In Nh3 Ligand Gains Formal Positive Charge As N In Ammonium Ion Chemistry Stack Exchange
0 Response to "Charge of Nh3 Ligand"
Post a Comment